 |
| [Photo source : Shutterstock] |
Gene therapy is a treatment applied in a health condition associated with a genetic problem. While the therapy does not work in every patient, some individuals can gain benefit from it. That is why the US Food and Drug Administration released a new policy draft to help advance the studies of gene therapies.
FDA Reshaping Regulation for Gene Therapy
In the past year, the agency noticed the advancement of three different gene therapy products that showed the rapid development in the field. They acknowledged the potential of gene therapies in treating numerous debilitating diseases, such as Huntington’s disease, hemochromatosis, and cystic fibrosis. These therapies may also help non-genetic diseases like autoimmunity, cardiovascular problems, and even HIV/AIDS.
So, FDA made efforts to modernize the approach in dealing with specific aspects of gene therapy development. The modernization of the regulation is also helpful for companies focused in this field of study. Right now, a draft of new policies has covered six aspects of gene therapy:
1. Gene Therapy for Hemophilia: Hemophilia is a rare disorder in which the blood does not clot normally. The abnormally is caused by a genetic mutation in the proteins linked to the blood clotting process. Current gene therapies for the disorder are often given in single-dose regimens to provide a long-term production of abnormal or missing clotting proteins. The FDA draft includes considerations of efficacy, safety, study design and population, and patient experience for every clinical trial. The draft also covers the recommendations concerning surrogates.
2. Gene Therapy for Retinal Disorders: Disorders of the retina can seriously affect the patient’s eyesight. If left untreated, the disorders can cause permanent blindness. One of many genetic retinal disorders is vitelliform macular dystrophy, wherein a degeneration occurs in the macula. The FDA draft considers the development of gene therapies for both pediatric and non-pediatric patients. The draft also includes different approaches of gene therapy for retinal disorders, such as intravitreal injections, subretinal injections, and device implantation.
3. Gene Therapy for Rare Disorders: There are rare diseases that exist in the world and these conditions affect at least 200,000 people in the US alone. According to Global Genes, an estimated 7,000 rare diseases affect more than 300 million people in the world, and about 80 percent of these disorders are triggered by a single genetic problem. Osteogenesis imperfecta and Von Hippel Lindau disease are a couple of known rare disorders. The FDA draft will provide the missing guidelines for approved therapies of rare diseases. The recommendations cover manufacturing, preclinical trial, and clinical trial design of future therapies.
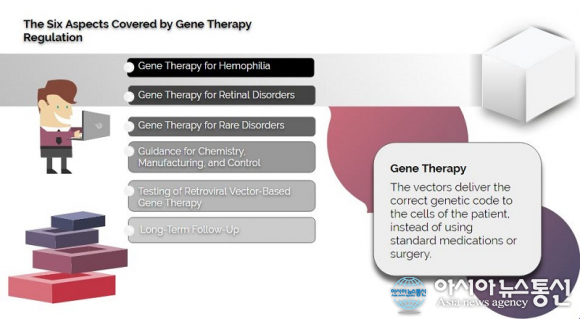 |
4. Guidance for Chemistry, Manufacturing, and Control: This recommendation gives manufacturers and sponsors the required information about the identity, potency, purity, quality, and safety of experimental gene therapies. Also, the policy affects the application of a drug or device and the combination of drugs or drug and device in treating genetic disorders.
5. Testing of Retroviral Vector-Based Gene Therapy: At the moment, viruses are the most common vectors of gene therapies. These viral vectors allow molecular biologist transport the therapeutic genetic code to patients. The FDA offers additional recommendations for proper testing of viral vectors in gene therapies. It includes proper identification of the vectors and the amount to be tested.
6. Long-Term Follow-Up: In clinical trials and observational studies, a long-term follow-up must be conducted in patients to determine the efficacy, safety, and adverse effects of experimental gene therapies. The FDA gives recommendations on the designs of long-term follow-up. Not only LTFU recommendations support data gathering, it also protects study participants and researchers behind the study.
According to the FDA, if these guidelines are finalized, it will replace the April 2008 CMC policies and November 2006 RCR and LTFU guidance.
“Gene therapy represents one of the most promising opportunities for developing highly effective and even curative treatments for many vexing disorders. Some of these products are almost certainly going to change the contours of medical practice, and the destiny of patients with some debilitating diseases,” stated Dr. Scott Gottlieb, commissioner of the FDA.
About Gene Therapy
In gene therapies, the vectors deliver the correct genetic code to the cells of the patient, instead of using standard medications or surgery. According to the National Institutes of Health, the known approaches of gene therapies include the replacement of mutated genetic code with a healthy copy, the deactivation of the mutated genetic code, and the introduction of a new genetic code to assist the body in fighting the disease.
Last year, a gene therapy for hemophilia successfully worked among 10 study participants. The viral vector has been administered intravenously to deliver the factor IX blood clotting protein. After 18 months, a follow-up has been conducted and found that the participants were able to produce factor IX. Their livers produced about 34 percent normal of the clotting protein production rate of factor IX, according to Science Mag.
 |
| [Photo source : Shutterstock] |

